May 2021 Volume 32, Issue 5
|
|
Get involved for International Batten Disease Awareness Day!
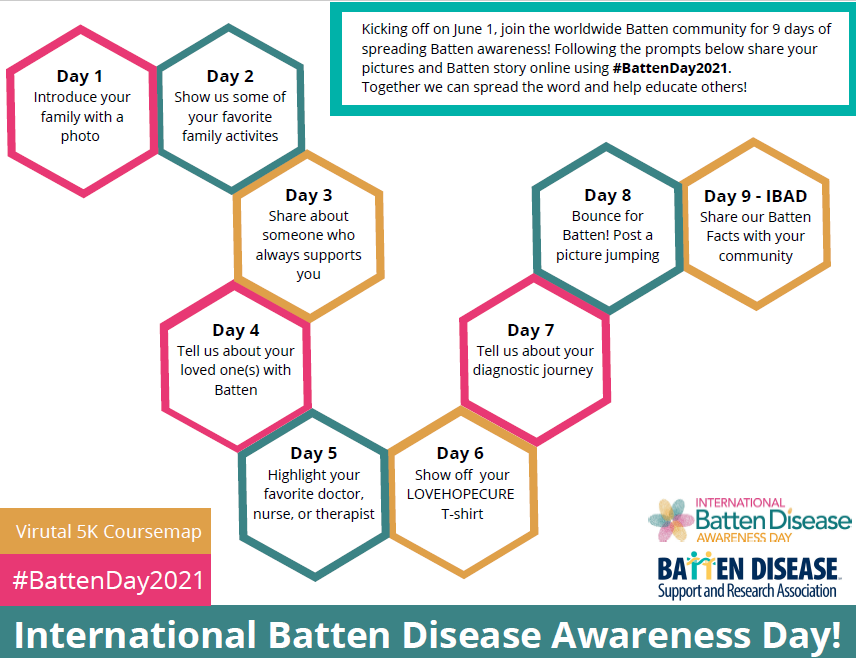
For International Batten Disease Awareness Day, we are raising awareness with an activity coursemap of 9 days of activities kicking off June 1st, ending with the Virtual 5k Run/Walk on June 9th!
Registration for the International Batten Disease Awareness Day Virtual 5k Run/Walk is live! To register, visit: https://web.charityengine.net/IBAD2021
Click HERE to see the official Facebook page for more information!
Check out the coursemap below for the event details!
|
|
BDSRA is looking to add parent and non-parent board seats!
Applications are due August 15th
To see a full description of roles and responsibilities please click here.
Note from Board Member Barb Wuebbels about her
journey to the BDSRA Community:

I first became aware of Batten’s disease in early 2012 at a meeting for the clinical and scientific teams at BioMarin. At that meeting they showed videos of the dogs at the University of Missouri who were being treated with Brineura and the Noah’s Hope video. I remembering coming away proud that I worked for a company that was developing a treatment for such a devastating disease.
In preparation for the trial’s start I traveled to Germany and Argentina to meet with leading researchers. I also traveled to London to meet with a group of Batten’s parents and the British MPS Society, who provided the logistical support for the clinical trial in Europe. I came away inspired by the dedication of everyone involved to make the trial happen as quickly and safely as possible.
That summer I went to my first family conference in Nashville. (There was a terrible thunderstorm and all the power at the hotel went out! There was only one elevator that worked and so we all had to hang out in the lobby till the power came back on a while later.) There I started to meet Battens families and their children. The thing that impressed me the most was that bereaved parents continued to come to the annual meetings to offer support to new families. In the other family organization’s’ I had worked with there was no place for families after their loved one died. I was so impressed that families were “lifers”- once they came to the BDSRA community they stayed long after their children became angels. I was also moved by the memorial service where every child who had passed was remembered by name and picture. The environment made it comfortable for parents to share memories, ask questions, and support each other.
Later that year as I was leaving BioMarin I talked to Marge Frazier about ways to stay involved with the community. I was reluctant to leave BioMarin because I would lose touch with the BDSRA community. She suggested I run for the Board. I did and gratefully I was elected!
Dave Pearce, Kate Haller and I are the only members of the board who do not have affected children. I think we provide legal guidance, insight into the drug development process, important scientific advances, and key contacts. We think of ourselves as “lifers” too because we love the Batten’s community.
This past year I have been a member of the Executive team which has allowed me to be more involved with the community. Switching to virtual family meetings and significant changes in the staff have made it a challenging year. I feel we are a much healthier organization today.
I am grateful to be part of this amazing community and look forward to being able to see everybody in person soon. The board meets “in person” in January and at the summer family conference. I look forward to learning more about the Batten’s community and ways in which the Board can help it succeed at these meetings.
If you have thought about joining the Board I would encourage you to do so. We need everyone’s talents and experience to improve the organization. New board members will join in January, 2022. We always meet somewhere warm which is a welcome break from winter. I hope you will join me there. Service to others is a great way to start the New Year.
|
|
Register for the Annual Family Conference by June 7th to secure your Conference Box & T-shirt!
|
|
We are looking for remote volunteers for
the Virtual Annual Family Conference!

|
|
Community Updates

The Fore The Journey Fund is a joint collaboration with the BDSRA and the ForeBatten Foundation open to all families regardless of income or need.This unique grant offers Batten families experiences, memberships, and gifts in the hopes of providing happy moments. Your request can be as individual as you! For this family, a trampoline brought huge smiles to the whole family and provided fun all summer long.
|
|
Resource Corner: Rare Disease Week on Capital Hill
Rare Disease Week on Capitol Hill brings together rare disease community members from across the country to be educated on federal legislative issues, meet other advocates, and share their unique stories with legislators. While we had hoped to host this event in-person, for the safety of all advocates, Rare Disease Week will be going virtual for 2021.
Virtual Rare Disease Week on Capitol Hill 2021 will be held July 14th through July 22nd and will include the same opportunities as in-person Rare Disease Week, plus more!
Registration is live! Visit:
https://everylifefoundation.org/rare-advocates/rare-disease-week/
|
|
Upcoming Events for Batten Families

Registration for the International Batten Disease
Awareness Day Virtual 5k Run/Walk is live!
CURRENT VIRTUAL 5K TEAMS REGISTERED:
ALLSTRIPES
AMELIA PALERMO
BIOMARIN
DAXX DESERVES
DAXX ROBAR
EVELYN
EXICURE
JON MILLER
LOVE FOR LIAM
MAXWELL BURNHAM
TAYLOR'S TALE
BEEDLE
EMMA
NZ
WEIMER LAB
|
|

BDSRA understands that each stage of this journey is different, and we are here for you. This is an opportunity for loved ones to gather, share and support one another. Please consider joining us at 7pm EST on June 24th, 2021. Register to attend by clicking the button below:
|
|
New dates for NCL 2021! The International Congress on Neuronal Ceroid Lipofuscinosis event will take place at Washington University, St Louis, October 6-10, 2021 and will be a 'hybrid' conference. This means both in-person and online-only registration options will be available.
This a parent-friendly conference; decoding the science for everyone to understand. BDSRA's Amy Fenton Parker and Morgan DeBoth will be in attendance. All are welcome!
More details can be found by visiting: www.ncl2021.org
|
|


The RARE Drug Development Symposium, in partnership with the Orphan Disease Center of the University of Pennsylvania , focuses on educating both beginners and advanced participants on the drug development process. Here, all members of the rare disease community are encouraged to explore their role in the rare disease drug development process in new and innovative ways. You can see highlights from the 2020 RARE Drug Development Symposium here, or discover what’s next in drug development in the RARE Drug Development 2020 Report.
Join us for this year’s virtual event, June 10-11, 2021, with an optional pre-conference workshop on June 9. Here you will explore the landscape of rare drug development, interact with experts, patients, and advocates in the field, and ultimately discover your role in advancing drug therapies and educating yourself on the complex world of rare disease drug development. Read more about exciting updates to this year’s event in our press release.
Interested in becoming a speaker for the RARE Drug Development Symposium, or want to recommend someone? Fill out the speaker inquiry form here.
Get a behind-the scenes preview of what some of our featured speakers will be presenting on during this year’s RARE Drug Development Symposium, here.
Check out more information by going to their website here.
|
|
Research Updates
Natural History Study enrolling virtually now, until it’s safe for families to travel.
https://clinicaltrials.gov/ct2/show/NCT03822650?cond=cln5&draw=2&rank=1
Caregiver Focus Groups for CLN5
Dates will be determined by the families’ availability. View article here.

Neurogene is currently working with RTI Health Solutions (RTI‑HS), an independent, nonprofit research organization to better understand the disease progression of Batten disease subtype CLN5 from the caregiver’s perspective.
Caregivers of children (either currently living or deceased within the past 12 months) with Batten disease subtype CLN5 who are eligible for this study (Qualitative Interviews With Caregivers of Children With CLN5-Specific Batten Disease) and live in the United States will be invited to participate in a telephone interview with two RTI‑HS interviewers lasting approximately 90 minutes. Interview participants will receive an electronic gift card (e.g., Visa, Amazon, or other online store of choice) for $150 in appreciation for their time.
During the interviews with RTI‑HS, caregivers will be asked to describe their child’s and their own experiences surrounding the journey to and following diagnosis, including observed signs and symptoms of the disease, delays in any of the developmental milestones, and perceived impacts of Batten disease subtype CLN5 on the child, caregiver, and family.
The discussion will be audio recorded and transcribed to ensure the interviewers capture the important points that participants share and to help write a report summarizing the results of the interviews. Participants’ names and any other identifying information will not be associated with the transcripts. The audio recordings will be destroyed once a final report is written.
If you are interested in participating in a telephone interview, please call Danielle at L&E Research at 720-647-3228 who will ask you a few questions to confirm that you qualify for this study. If you qualify, L&E Research will schedule you for an interview at a day and time that is convenient for you.
|
|

Taysha Gene Therapies is excited to share the opening of the manufacturing facility in Durham, NC. Sharon King, Taylor’s mom and President of Taylor’s Tale, highlighted what this means for the rare disease community and especially for CLN1 disease children. Please read Taysha’s letter to the community here.
|
|

On May 5, 2021, REGENXBIO provided an update on our two investigational gene therapies (RGX-181 and RGX-381), both for the treatment of CLN2 Batten disease.
RGX-181 for the Treatment of CNS manifestations of CLN2 disease
An Investigational New Drug (IND) application was submitted to the U.S. Food and Drug Administration (FDA), after which the FDA notified REGENXBIO in a
letter that its proposed trial had been placed on clinical hold and the agency requested more information to support the initial dose selection and certain study drug administration procedures. REGENXBIO is evaluating the FDA’s requests and plans to provide an update on the program in the second half of 2021.
RGX-381 for the Treatment of Ocular Manifestations of CLN2 disease
Based on communication with the FDA and the update from the RGX-181 program, REGENXBIO now expects to provide a program update for RGX-381 in the second half of 2021.
For more information on our CLN2 programs, please email: CLN2@regenxbio.com or PatientAdvocacy@regenxbio.com
|
|
Research Study Opportunity
-page-001.jpg)
-page-002.jpg)
|
|

May 2021 Facebook Fundraiser Spotlight:
We are so excited each day to log in and see the list of your names growing. Thank you for sharing and supporting our mission on social media! You can visit each fundraiser by clicking on the names above. Thank you!
|
|
In Loving Memory
Remember with us those we have lost to Batten disease. It's in their honor and memory that we work every day to build a brighter future for families.
Jamila Bengaliwalla
5/27/2016-5/19/2021
To have your loved one's name placed in the Illuminator please reach out to Morgan at mdeboth@bdsra.org.

|
|
Thank You BDSRA Community of Donors!

Thank you to the donors last month who gave so generously to honor and in memory of loved ones and our community as a whole. We want to acknowledge you for your contributions. They allow us to keep serving families and advancing the science of a meaningful treatment. Your dollars and support have helped create the leading patient organization in the Batten disease community, and we are dedicated to continue that legacy of children and families
You can view recent donations to BDSRA
by clicking here.
|
|
Your gift makes all the difference in the lives of families.
|
|